Science Notes
Chapter 1-Chemical Reactions and Equations
- Learn CBSE Questions
- Learn insta questions
- Chapter 1 Notes (Byjus)
- Edumantra Video
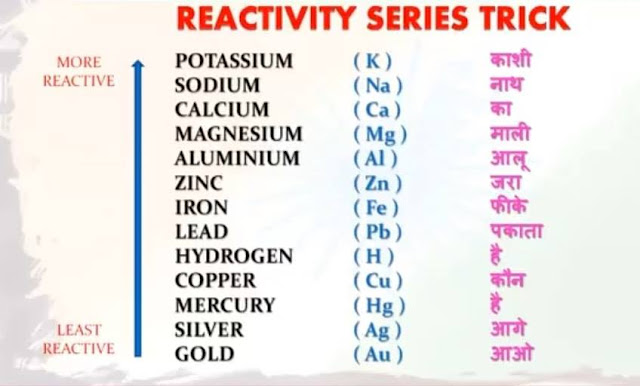
- Physicswallah (For reactivity series)
Chapter 2-Acids, Bases and Salts
- Learn CBSE Questions
- Learn insta questions
- Colgyan imp Questions
- Chapter 2 Notes (Byjus )
- Notes in PDF format
- Notes topper learning pdf
- Edumantra Video acid bases and salts
Chapter 3 Metal and Non Metal
- Aglasem pdf notes
- Learn CBSE Questions -1
- Learn CBSE QUESTIONS -2
- Learn insta questions 1
- Learn Insta questions 2
- Chapter 3 Notes (Byjus )
- Vidyakul pdf (optional)
- Edumantra Video metal and non metal
Chapter 4 CARBON AND ITS COMPOUNDS
Chapter 5 -Periodic classification of elements
Atomic number is the number of protons there in an atom.
Mass Number = Number of protons + Number of neutrons
The major difference between atomic number and mass number is that the atomic number states the number of protons present in an atom whereas, the mass number indicates the total of the number of protons and the number neutrons present in an atom.
A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature of chemistry.
The six commonly recognised metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. Five elements are less frequently so classified: carbon, aluminium, selenium, polonium, and astatine. On a standard periodic table, all eleven elements are in a diagonal region of the p-block extending from boron at the upper left to astatine at lower right. Some periodic tables include a dividing line between metals and nonmetals, and the metalloids may be found close to this line.
Which Elements Are Metalloids?
Following are the elements that are considered to be metalloids:
- Boron (B)
- Silicon (Si)
- Germanium (Ge)
- Arsenic (As)
- Antimony (Sb)
- Tellurium (Te)
- Polonium (Po)
These seven elements were classified as metalloids in the periodic table from the 13th to the 16th group.
It was supposed to classify Astatine as either a non-metal or metalloid. The formation of metals has also been expected.
Periodic Table Trends:
The following trend in periodic properties of elements is observed:
Atomic size Trends:
- The distance between the centre of the nucleus and the outermost shell of an atom is known as the atomic radius.
In a group the atomic size increases due to the addition of shells as we move from one period to another.
Across a period the atomic size decreases as the number of shells remain the same while the nuclear charge increases. This leads to the pulling of electrons from the outermost shell towards the nucleus thereby decreasing the size.
Metallic character Trends:
- The elements which lose electrons to form cations are known as metals.
Metallic character increases as we move down the group because the atomic size increases which lead to easy loss of electrons.
On the other hand, it decreases across a period as we move from left to right. This happens because there is an increase in nuclear charge which makes it difficult for an atom to lose electrons.
Non-metallic character Trends:
- The elements which have a tendency to gain electrons are known as non-metals.
The tendency to gain electrons increases on moving across a period due to an increase in the nuclear charge and decrease in the atomic size.
Hence, non-metallic character increases across a period.
As we move down the group the non-metallic character decreases due to increase in the atomic size.
Ionization potential Trends:
- Ionization potential is defined as the amount of energy required to remove an electron from the outermost shell of a gaseous atom and convert it into a positively charged gaseous ion.
The periodic properties in terms of ionization potential increase because the atomic size decreases across a period due to increase in the nuclear charge.
When we move down the group, ionization potential decreases due to the increase in atomic size.
Melting Point Trends:
- The melting point of an element is basically the energy required to change the state of an element from its solid state to its liquid state.
Which essentially implies breaking a few bonds. Thus, higher the stronger the bond between the atoms, higher will be the melting point. Let us look at the elements in the ascending order of their melting points.
Boiling Point Trends:
- Just like how the strength of the bonds between atoms affect the Melting Point, the boiling point depends on the heat energy required to create a transition from liquid to gaseous state.



















:max_bytes(150000):strip_icc():format(webp)/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
Comments
Post a Comment